ponman : A package for BioArgo data Analysis
Midhun Shah Hussain, Smitha B. R. & A. A. Mohamed Hatha
ponman is an R Language implementation developed to reduce the data-researcher barrier to promote the effective use of Bio-Argo floats. More than an R package it have comprehensive tool sets for the Bio-Argo, from data acquisition to plotting. ponman is a user-friendly package for a regular R user with the aid of our detailed documentation. Oceanographers usually cluttered with enormous amount of data, for analysis and for downloading is a tedious job. ponman for Bio-Argo could download the data direct to working directory according to your prior categories. ponman prefers netcdf(.nc) files, which is a set of software libraries and self-describing, machine-independent data formats that support the creation, access, and sharing of array-oriented scientific data. Extraction of the data from .nc files to readable form is the first thing one could do by ponman.
How to install ponman
As ponman was not officially launched in CRAN, it need to download from github. please follow the instructions
install.packages("devtools")
library(devtools)
install_github("mishahublog/BioArgo/ponman",ref = "ponman")
Get data to ponman
Getting data to ponman can attain in two ways. Since API programming is not available, the database can be access by FTP(File transfer protocol) or you can manually download to the working directory.
#extract data from a location and time
extractlocation_time<- get_data2_ponman_(mode = "geotime",location = "indian_ocean",year = "2010",month = "02")
You will get an output with a choice to download multiple to single files
get_data2_ponman_(mode = "geotime",location = "indian_ocean",year = "2010",month = "02")
[1] "20100201_prof.nc" "20100202_prof.nc" "20100203_prof.nc" "20100204_prof.nc" "20100205_prof.nc" "20100206_prof.nc"
[7] "20100207_prof.nc" "20100208_prof.nc" "20100209_prof.nc" "20100210_prof.nc" "20100211_prof.nc" "20100212_prof.nc"
[13] "20100213_prof.nc" "20100214_prof.nc" "20100215_prof.nc" "20100216_prof.nc" "20100217_prof.nc" "20100218_prof.nc"
[19] "20100219_prof.nc" "20100220_prof.nc" "20100221_prof.nc" "20100222_prof.nc" "20100223_prof.nc" "20100224_prof.nc"
[25] "20100225_prof.nc" "20100226_prof.nc" "20100227_prof.nc" "20100228_prof.nc"
Do you want download all these files?
1: Yes
2: No
Selection:
You can take single files by just typing name without “”
Selection: 2
Type the file name you want to download: 20100226_prof.nc
Reading single files
After downloading the data-sets in working directory. It could be simply read by the function “ExtractBioArgo”
Readprofile_1 <- ExtractBioArgo(bioarg = "2902092_001_20180531065847123.nc")
Horray!!, All paramters available
Date latitude longitude cycle.no pressure temperature salinity oxygen chlorophyll backscatter
1 2013-02-24 19.08436 66.54972 1 0.6 26.051 36.294 182.54312 -0.02920 0.00028
2 2013-02-24 19.08436 66.54972 1 1.6 26.050 36.298 182.45311 0.31755 0.00040
3 2013-02-24 19.08436 66.54972 1 2.8 26.049 36.299 182.45979 0.29930 0.00108
4 2013-02-24 19.08436 66.54972 1 3.6 26.054 36.295 182.31361 0.29200 0.00107
5 2013-02-24 19.08436 66.54972 1 4.4 26.054 36.296 182.23773 0.32850 0.00116
6 2013-02-24 19.08436 66.54972 1 5.2 26.053 36.297 182.25000 0.32850 0.00104
7 2013-02-24 19.08436 66.54972 1 6.5 26.052 36.298 182.19359 0.31755 0.00108
8 2013-02-24 19.08436 66.54972 1 7.8 26.053 36.298 182.06905 0.30660 0.00104
9 2013-02-24 19.08436 66.54972 1 8.6 26.053 36.298 182.03868 0.32850 0.00116
10 2013-02-24 19.08436 66.54972 1 9.6 26.052 36.299 182.01314 0.31390 0.00097
11 2013-02-24 19.08436 66.54972 1 12.7 26.053 36.298 181.91534 0.35916 0.00128
12 2013-02-24 19.08436 66.54972 1 17.5 26.054 36.299 182.02058 0.42121 0.00109
[ reached getOption("max.print") -- omitted some rows ]
Batch processing of multiple files
Reading multiple files are very convenient for section graphs and comprehensive analysis. To attain this we need to make list of profiles. Here we are going to make a list of profiles from 2013 of platform 2902092.
####Before execuiting the codes be sure about the working directory, check your datasets there.
list.files()
[1] "2902092_001_20180531065847123.nc" "2902092_002_20180531065955638.nc" "2902092_003_20180531070031783.nc"
[4] "2902092_004_20180531070106448.nc" "2902092_005_20180531070157113.nc" "2902092_006_20180531070239736.nc"
[7] "2902092_007_20180531070456557.nc" "2902092_008_20180531070528935.nc" "2902092_009_20180531070606146.nc"
[10] "2902092_010_20180531070643511.nc" "2902092_011_20180604072048784.nc" "2902092_012_20180604072134870.nc"
[13] "2902092_013_20180604072205241.nc" "2902092_014_20180604072235039.nc" "2902092_015_20180604072258139.nc"
[16] "2902092_016_20180604072347083.nc" "2902092_018_20180604072511656.nc" "2902092_019_20180604072543416.nc"
[19] "2902092_020_20180604072837202.nc" "2902092_021_20180604081553398.nc" "2902092_022_20180604081620427.nc"
[22] "2902092_023_20180605150436103.nc" "2902092_024_20180605150531084.nc" "2902092_025_20180605150603553.nc"
[25] "2902092_026_20180607052540419.nc" "2902092_027_20180607052622437.nc" "2902092_028_20180607052724234.nc"
[28] "2902092_029_20180607052801723.nc" "2902092_030_20180607052832895.nc" "2902092_031_20180607052859008.nc"
[31] "2902092_032_20180607052923673.nc" "2902092_033_20180607052942654.nc" "2902092_034_20180607053022688.nc"
[34] "2902092_035_20180607053230011.nc" "2902092_036_20180607053713007.nc" "2902092_037_20180629043808803.nc"
[37] "2902092_038_20180629043907204.nc" "2902092_039_20180629043957566.nc" "2902092_040_20180629044036565.nc"
[40] "2902092_041_20180629044119761.nc"
save your list by giving a name and execute “batch” function
profile_list2013<- list.files()
batch(profile_list2013)
of course you need to give a name to batch function too
> profiles2013<- batch(profile_list2013)
Horray!!, All paramters available
Horray!!, All paramters available
Horray!!, All paramters available
Horray!!, All paramters available
Horray!!, All paramters available
Horray!!, All paramters available
Horray!!, All paramters available
Horray!!, All paramters available
Horray!!, All paramters available
Horray!!, All paramters available
Horray!!, All paramters available
Horray!!, All paramters available
Horray!!, All paramters available
Horray!!, All paramters available
Horray!!, All paramters available
Horray!!, All paramters available
Horray!!, All paramters available
Horray!!, All paramters available
Horray!!, All paramters available
[ omitted some rows ]
There will be a notification if all the parameters are available for each profiles
“Horray!!, All parameters available”
plotting BioArgo profiles
Now we have the data in hand in list form. you could browse each profiles(or each cycles) by adding numbers in double square brackets
profiles2013[[1]]
You could plot single profiles by simply type
plot_BioArgo(profiles2013[[1]])
 This is an overview, actually you can plot single profiles also, explore the options!!
By the way you can plot your desired cycles/profiles.
This is an overview, actually you can plot single profiles also, explore the options!!
By the way you can plot your desired cycles/profiles.
##Triming and filtering of data for plotting
After plotting your first overview(showned above), you could trim accordingly. Mostly we want to get specific depths or in some selected dates. This could be possible by “Filter_bioArgo()”
Suppose you want to trim all the profiles to 0 to 300 pressure values
Filter_bioArgo(batchlist = profiles2013,parameter = "pressure",start = 0,end = 300)
Give a name and plot
profiles2013_0to100<- Filter_bioArgo(batchlist = profiles2013,parameter = "pressure",start = 0,end = 300)
plot_BioArgo(profiles2013_0to300[[1]])
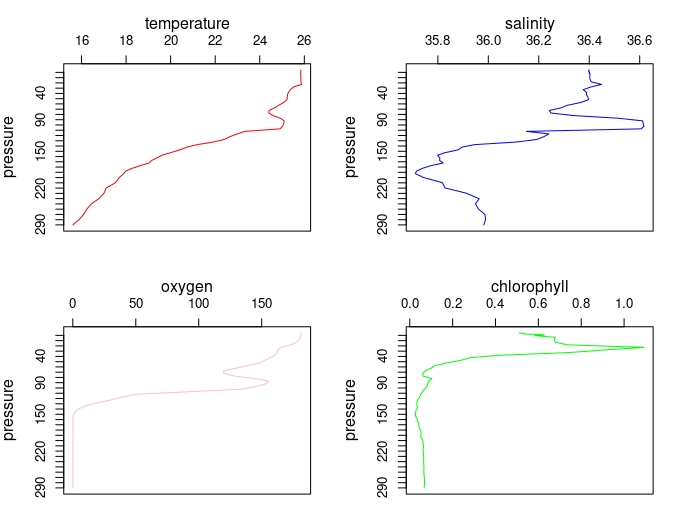
Contour Graphs for comprehensive ocean studies
Section plots helps to interpolate multiple profiles over a spatial or temporal section. As BioArgo floats are not linear in a spatial scale(due to drifting). The possible way here applied was the plotting of temporal sections.Lets start with temperature. Be sure that you data is in list, if it is not , make it using “batch()” as done above. The function Contour.BioArgo() will done this perfectly.
# In a simple way the function provides an overview
Contour.BioArgo(Argolist = profiles2013)

You could also plot single paramters. check the available paramters by ? Contour.BioArgo
Contour.BioArgo(Argolist = profiles2013,parameter = "salinity",overview = FALSE)

Try changing its colour and levels using the same arguments of base contouring in R, you could also change every arguments as your wish like the default contour function.
#changing colour
Contour.BioArgo(Argolist = profiles2013,parameter = "salinity",overview = FALSE,col="red")
#changing Level
Contour.BioArgo(Argolist = profiles2013,parameter = "salinity",overview = FALSE,col="red",nlevel=50)

The contour plots will follow most of the arguments of native contour function
Filled contours and contour overlays.
Filled contours are efficient ways to display overlays. Inaddition it also calculate Mixed layer Depth(MLD), there is an option to annotate it on the plots. Simply you could plot a filled contour by the function “Filledsectionplots()”
# You could any colour schemes, Here I used colourRamps's matlab.like() method
Filledsectionplots(Argolist = profiles2013,parameter = "temperature",col.val = matlab.like(30))

There is a list of possible overlays, which you could found by ?Filledsectionplots(). Here you could found the filled temperature with overlay of salinity.
Filledsectionplots(Argolist = profiles2013,parameter = "temperature",col.val = matlab.like(30),overlay = "salinity")

You can also use the trimmed data. here I am going to use the same data of above profile.
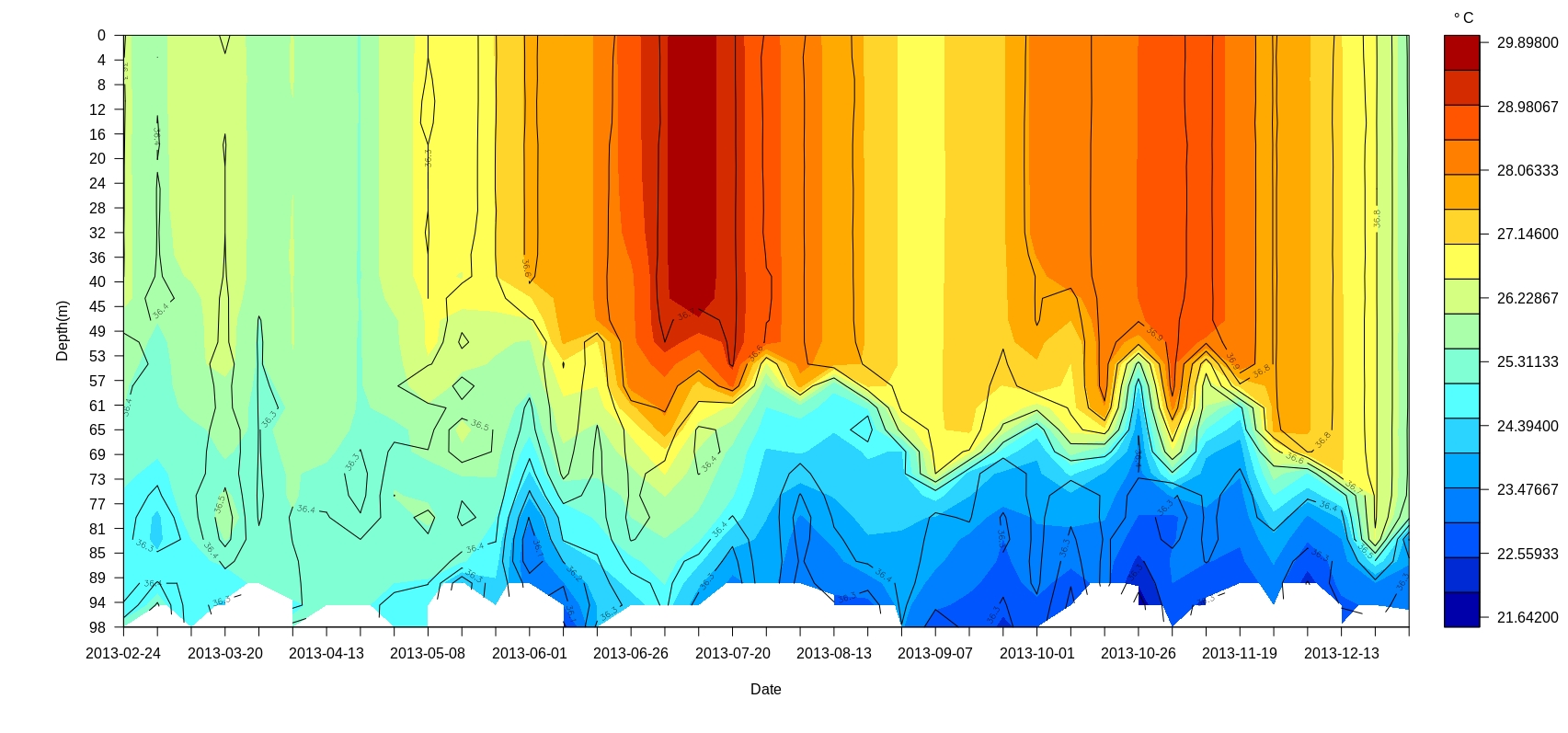
##Derived parameters
ponman also generate derived parameters such as density(), Mixed Layer Depth (MLD-m) and buoyancy frequency(N2) using the R package “gsw”. These parameters can be contoured as an overlay on filled contour of any basic parameter(Fig7). More additions of derived parameters in ponman are in progress.
Filledsectionplots(Argolist = profile2013,parameter = "chlorophyll",col.val = matlab.like(30),overlay = "N2")
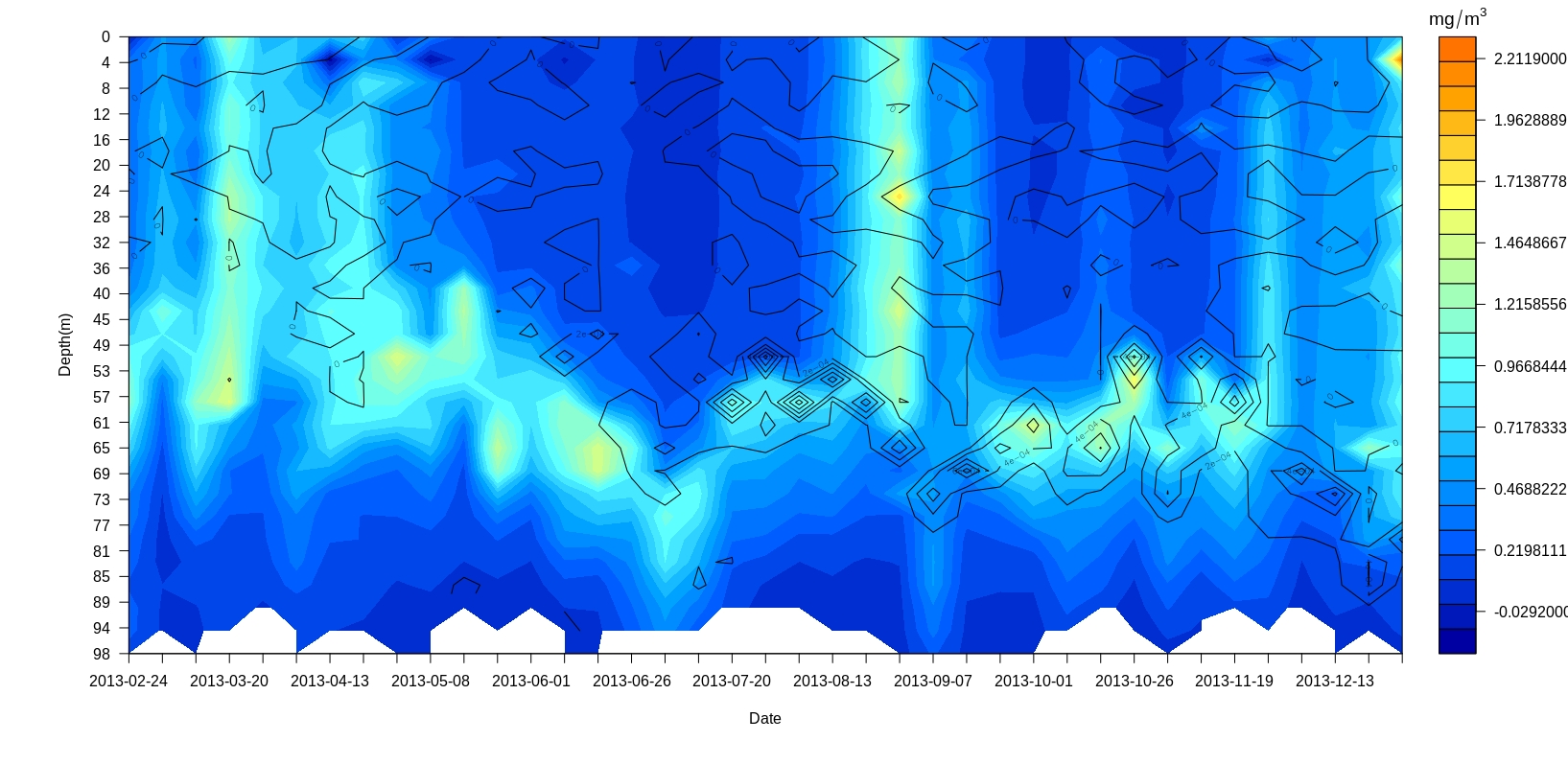
Mapping Argo floats locations
Mapping options in ponman includes plotting Argo trajectories,along with surface maps embedded with remote sensing data in the relevant time domain. Embedding remote sensing data using ponman needs more improvements from the current version, which needs to download in the working directory before plotting.Besides, the native arguments of R plots such as changing colors or line widths are still possible. A few examples are given below
Sat_bioArgo(batchlist = profile2013,
satdata = "plots/RSdata/A20030602018090.L3m_MC_CHL_chlor_a_4km.nc",# Adding remote sensing data
satdata.type = "chl",# Mention the type
satland ="grey",#Land colour
legend.month = TRUE)# Adding legends with month
Argo float trajectory with sea surface chlorophylls | Argo float trajectory with Sea Surface Temperature(SST)
:—————————:|:——————————:
 |
| 
Future scope of ponman
The same way ponman can add SST and PAR data from Aqua MODIS. Currently the datasets are need to be download seperately for overlay analysis. In near future, the data aquisition will also be add.We promised to add more features with more efficient object oriented programing(OOP). By the way,we are looking forward more suggestions from user base. Also we suggest to all users those who pursuing publications are requested to cite ponman, get access the format as follows.
citation(ponman)